ISO 13485 – Medical Devices Quality Management
Achieve Global Compliance in the Medical Device Industry
ISO 13485:2016 is the internationally recognised standard for quality management systems in the medical device industry. Whether you're a manufacturer, supplier, distributor, or service provider in the healthcare sector, achieving ISO 13485 certification demonstrates your commitment to quality, patient safety, and regulatory compliance.
At IMSM, we make ISO 13485 implementation simple, streamlined, and aligned with your business goals — helping you meet the rigorous demands of medical device regulations globally.

What is ISO 13485?
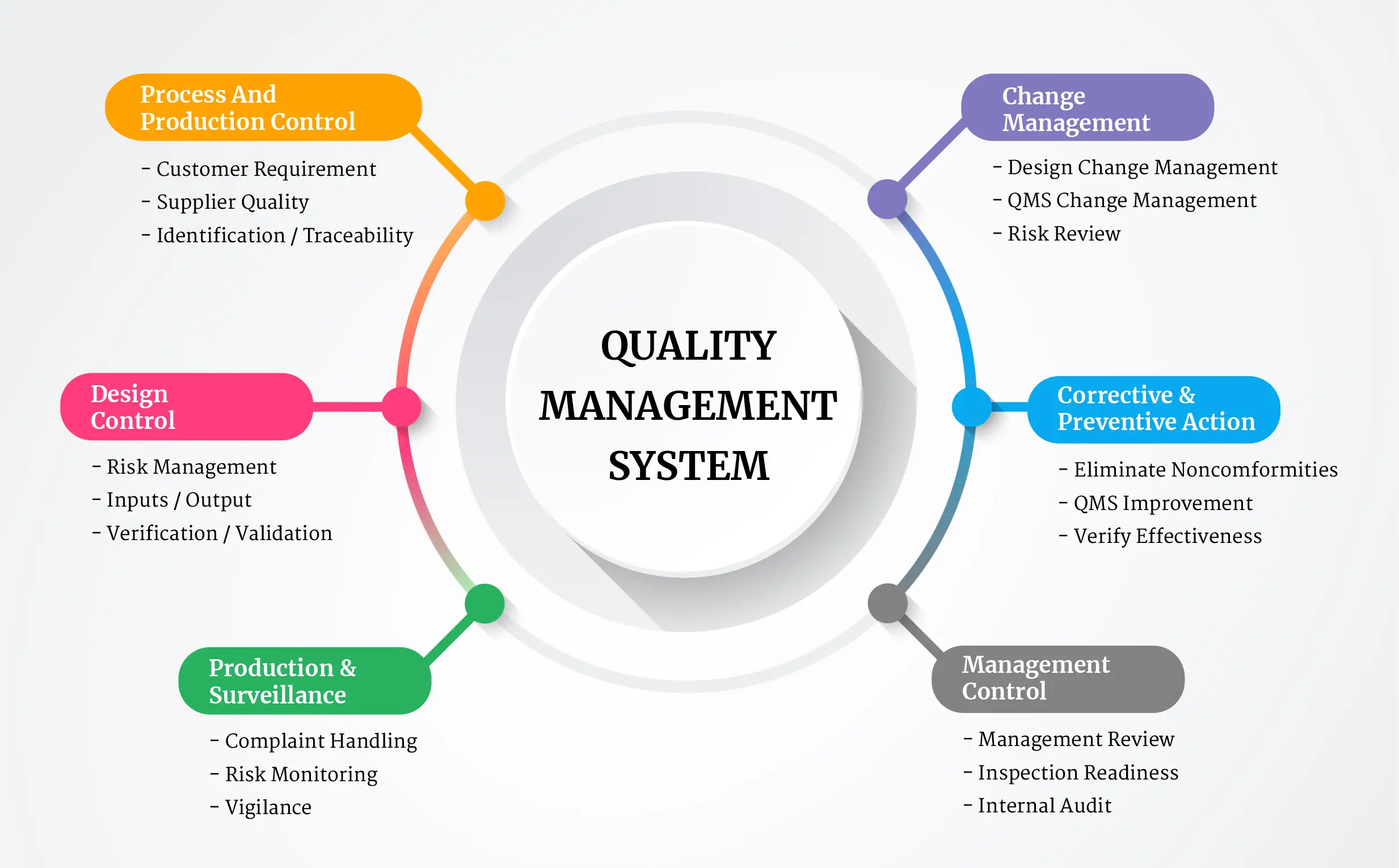
ISO 13485:2016 specifies the requirements for a comprehensive quality management system (QMS) for the design, development, production, installation, and servicing of medical devices and related services.
It is designed to work in alignment with international regulatory requirements such as the EU MDR (Medical Device Regulation), FDA 21 CFR Part 820, and other regional medical device laws.
Benefits of ISO 13485 Certification
Partnering with IMSM to achieve ISO 13485:2016 delivers a wide range of operational and competitive advantages:
-
Regulatory Compliance
Meet international regulatory requirements including EU MDR, UKCA, MDSAP, and FDA standards. -
Market Access
Gain faster entry into global markets and qualify for medical device tenders and contracts. -
Improved Product Quality and Traceability
Ensure consistent quality, safety, and effectiveness throughout the product lifecycle.
-
Risk Management and Patient Safety
Identify and control risks at every stage of your supply chain and production process. -
Enhanced Operational Efficiency
Standardise procedures, reduce defects, and streamline compliance documentation. -
Customer Confidence
Demonstrate your commitment to quality assurance and patient safety to clients, regulators, and healthcare providers.
Why Choose IMSM for ISO 13485?
IMSM’s ISO 13485 Implementation Process
We simplify the path to ISO 13485 certification with a proven and practical step-by-step process tailored to your medical device business:
Initial Consultation
We begin by identifying your business objectives, regulatory landscape, and product types. This ensures the ISO 13485 implementation is tailored to your specific requirements and business model.
Gap Analysis & Planning
Our expert consultants assess your current QMS against ISO 13485 standards. We then create a roadmap to close the gaps, aligned with applicable regional regulations.
Quality Manual & Documentation Development
We support you in creating or refining your quality manual, standard operating procedures (SOPs), risk management files, and product lifecycle documentation — all critical for ISO 13485 certification.
Staff Training & System Integration
We train your employees on ISO 13485 principles, document control, traceability, and regulatory reporting. Our aim is to embed a culture of quality throughout your organisation.
Internal Audit & Management Review
Before the official audit, we conduct internal reviews to ensure your systems are fully compliant and ready for certification.
External Certification Audit
We support you through the final audit process with a third-party, accredited certification body. After a successful audit, your business is awarded ISO 13485 certification.
Start Your ISO 13485 Certification Journey Today
ISO 13485:2016 is more than just a standard — it’s your ticket to international growth, improved product quality, and customer trust in a highly regulated sector.